
Shortly after that, the cotton bud or Q-tip containing DMG and ammonia can be seen being rubbed against the surface of a coin. Immediately, an insoluble bright red solid called nickel dimethylglyoxime, Ni(C 4H 8N 2O 2) 2, precipitates out of solution. The video shows a few drops of DMG, C 4H 6(NOH) 2, being added to a dilute solution of nickel II sulfate with a few drops of ammonia solution.
#Dmg reaction with nickel how to
The best course of action to prevent an allergic reaction is to avoid contact with products containing nickel and in this post we will to show how to make a cheap and quick nickel detection device at home or in the lab based on the reaction described above.Ī video describing how to test for the presence of nickel in various coins using such a device can be watched below (no sound). It is not known why people develop allergies to nickel and there is no cure. especially when worn for extended periods of time.
#Dmg reaction with nickel skin
In contrast, nickel plated jewellery will release vast amounts of nickel ions in contact with sweat from the skin and from everyday brushing, friction, abrasion, etc. For example, alloys such as many stainless steels contain nickel but do not release sufficient amounts of nickel ions to cause someone to become nickel sensitized or have nickel ACD reactions if they are already sensitive to nickel. Only a certain amount of released ions will cause a reaction. It is believed that the acids present in sweat dissolve a little of the nickel and it is the release of nickel ions that are responsible for causing nickel sensitization and ACD 4 that can accentuate conditions such as skin erythema, eczema, etc. A red itchy rash develops on the skin that has been in contact with the metal. This contact allergy or allergic contact dermatitis (ACD) usually flares up when sweating. for the benefit of people that suffer from a kind of skin sensitivity or dermatitis called Nickel itch.

The same procedure can be easily applied to metal objects that regularly come in contact with the skin, for example coins, jewelry, earrings, spectacle frames, watch straps, etc. This reaction is very sensitive and can be used as a confirmation test for the presence of nickel II cations even in very low concentrations. The reaction involves two dimethylglyoxime molecules acting as chelating agents to form the nickel dymethylglyoxime square planar complex. Both qualitative and gravimetric determinations of nickel are part of many chemistry courses. This reaction is still very much in use today for the detection of nickel metal ions due to its striking colour formation.
#Dmg reaction with nickel manual
Ni 2+ (aq) + 2C 4H 8N 2O 2 (aq)→ Ni(C 4H 7N 2O 2) 2 (s) + 2H + (aq)įigure 1: Structural equation from The Gravimetric determination of Nickel, Truman State University CHEM 222 Lab Manual 3 The balanced ionic equation for this reaction:


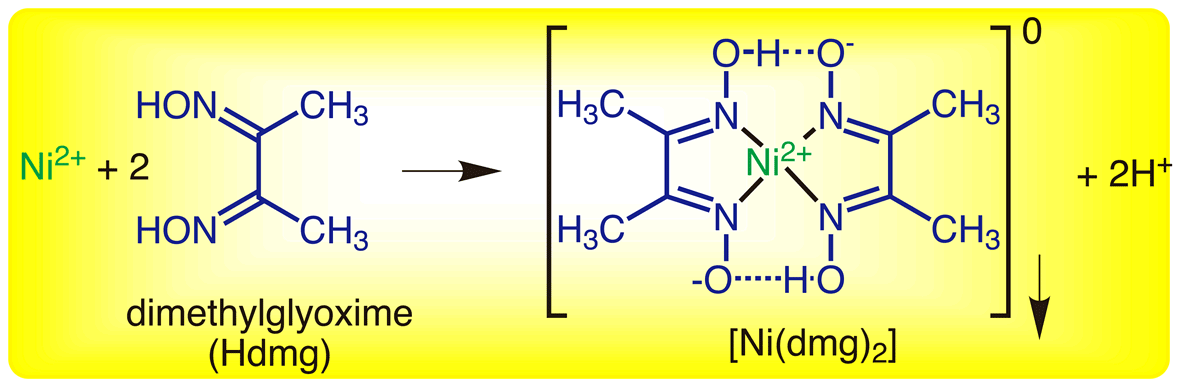
The chelation reaction of nickel ions with the organic bidentate ligand dimethylglyoxime (DMG) 1 in an alkaline ammonia medium producing nickel dimethylglyoxime, Ni(DMG) 2, a red cherry or raspberry colour precipitate has been known since 1905 when it was discovered by Russian chemist Lev Aleksandrovich Chugaev (see figure 1). It was the first organic spot test reagent used to detect a metal ion and as a result DMG is known as Chugaev’s reagent.


 0 kommentar(er)
0 kommentar(er)
